Edelife XLHED Clinical Trial
Now Enrolling Pregnant Women
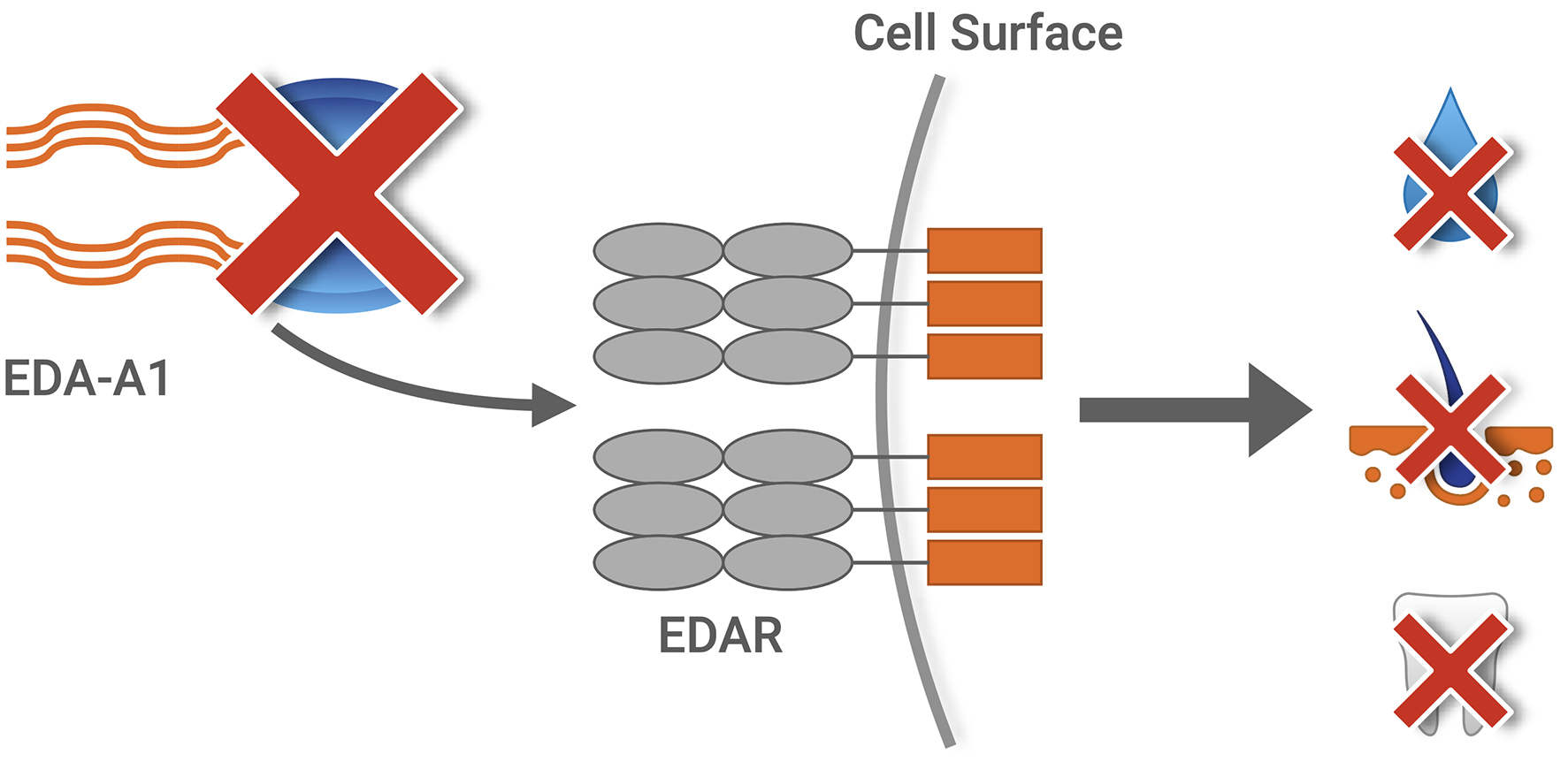
X-Linked Hypohidrotic Ectodermal Dysplasia (XLHED), the most common form of ectodermal dysplasia, is caused by a broad spectrum of mutations in the ectodysplasin A1 (EDA-A1) gene. The main symp- toms of XLHED are hypo- or anhidrosis, oligo- or anodontia and hypotrichosis. Current treatment options are limited to managing disease symptoms and complications. Effective corrective treatment for XLHED remains a high unmet medical need.
Edelife is a clinical study of the safety and possible health benefits of an investigational medicine for pregnant women expecting an ectodermal dysplasia (XLHED) affected boy.
Investigational Protein Intended to Replace EDA-A1 in utero
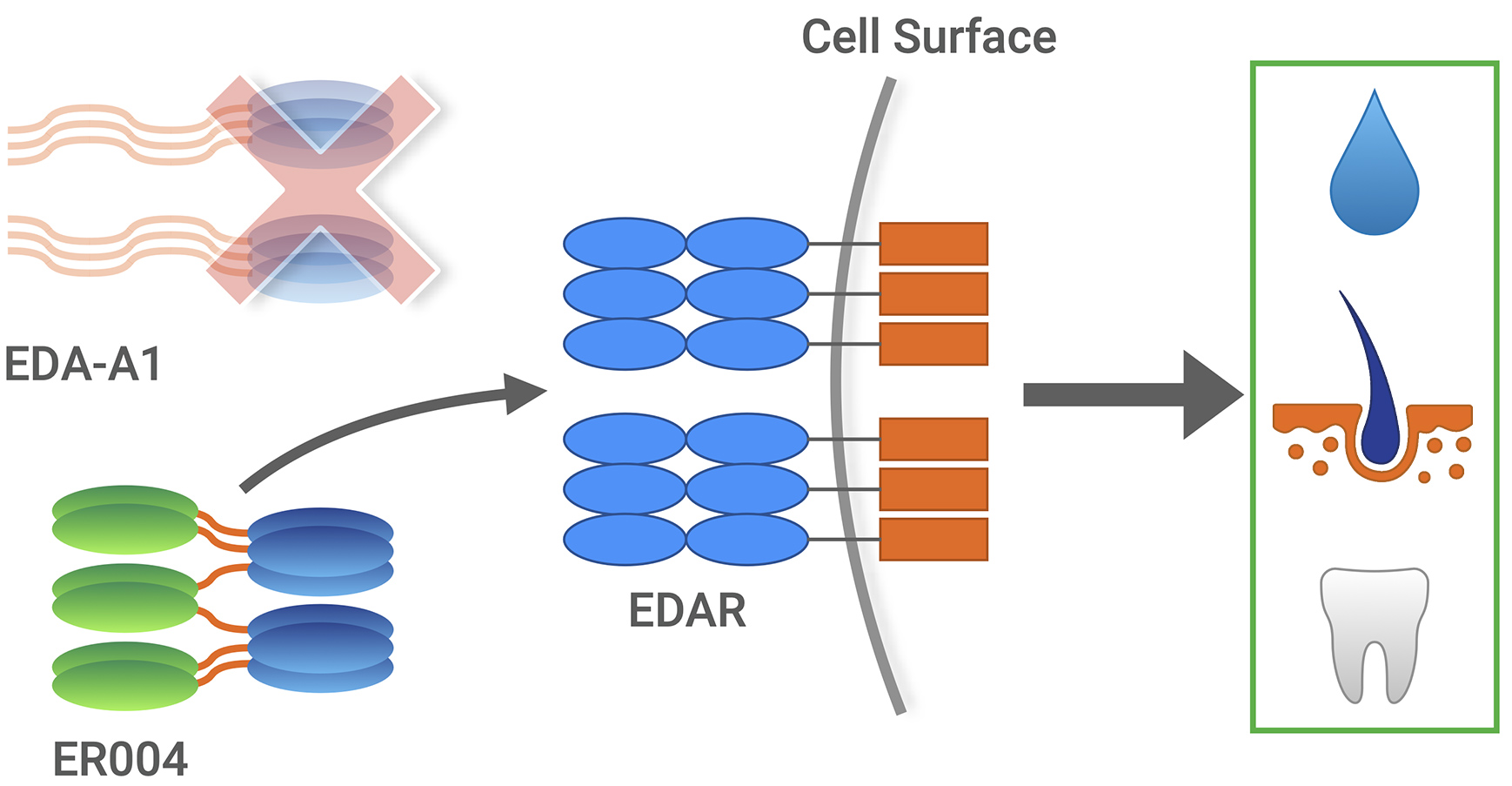
The Edelife investigational treatment, ER004, consists of a signaling protein replacement molecule designed for specific, high affinity binding to the endogenous receptor, intended to replace missing EDA-A1 protein in utero for patients with XLHED.
Administration at several specific points in pregnancy is essential, as ER004 impacts development of ectodermal precursors of sweat glands, teeth, etc.
EDA-A1 is missing in XLHED
ER004 as an intended replacement for EDA-A1
Compassionate Use of ER004 in XLHED Patients
Compassionate Use of ER004 in XLHED Patients
- Results from three XLHED-affected boys treated in utero with ER004 were first reported in the New England Journal of Medicine in 2018.1
- Long-term results (N=6 patients, follow-up range 2 to 6 years) were recently published in the International Journal of Molecular Sciences in 2023.2
- The aim of the Edelife trial is to confirm the safety and efficacy of ER004.
NEXT STEPS: EDELIFE STUDY ENROLLMENT


